DR P. MARAZZI/SCIENCE PHOTO LIBRARY
Open access article
The Royal Pharmaceutical Society has made this article free to access in order to help healthcare professionals stay informed about an issue of national importance.
To learn more about coronavirus, please visit: https://www.rpharms.com/resources/pharmacy-guides/wuhan-novel-coronavirus
Source: DR P. MARAZZI/SCIENCE PHOTO LIBRARY
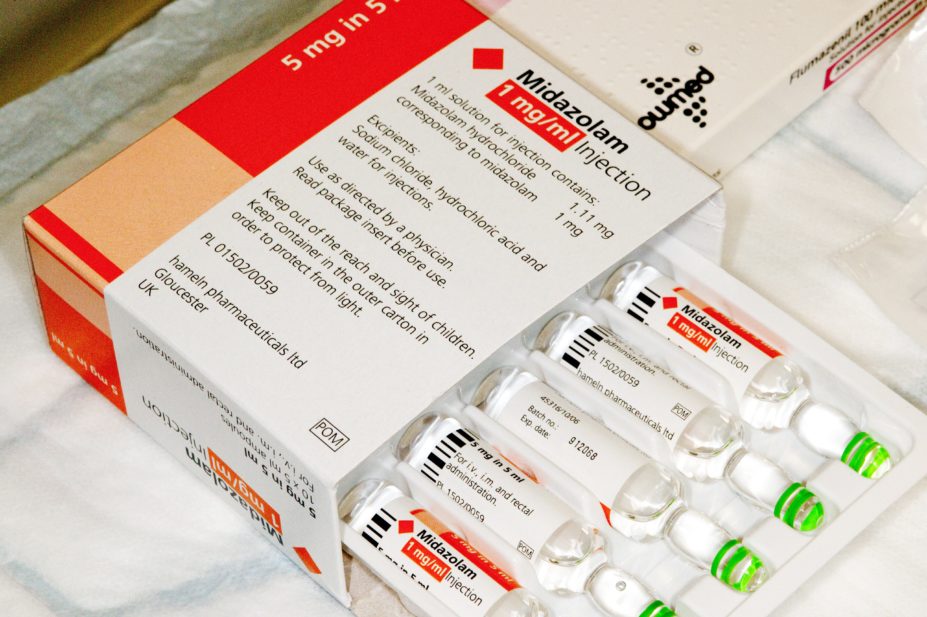
Midazolam is used to sedate patients with COVID-19 before intubation
Supplies of the sedative midazolam have been diverted from France as a “precaution” to mitigate potential shortages in the NHS caused by COVID-19, the Department of Health and Social Care (DHSC) has told The Pharmaceutical Journal.
A spokesperson from Accord Healthcare, one of five manufacturers of the drug, told The Pharmaceutical Journal that it had to gain regulatory approval to sell French-labelled supplies of midazolam injection to the NHS, after having already sold two years’ worth of stock to UK wholesalers “at the request of the NHS” in March 2020.
The DHSC said the request for extra stock was part of “national efforts to respond to the coronavirus outbreak”, which included precautions “to reduce the likelihood of future shortages”.
Midazolam is listed by the Royal College of Anaesthetists as a “first-line” sedative in the management of COVID-19 patients, and warns in guidance published on 2 April 2020 that it “may be subject to demand pressure”.
Matt Hancock, the UK health secretary, told the House of Commons Health and Social Care Select Committee on 17 April 2020 that intensive therapy unit medicines — including midazolam — are part of “a delicate supply chain” as they “are made in a relatively small number of factories around the world”.
While the DHSC confirmed that midazolam is still available to both primary and secondary care, it added that some suppliers of the sedative had limited or no stock availability.
A spokesperson from Accord Healthcare told The Pharmaceutical Journal on 11 May 2020 that it was out of stock of midazolam injection after the NHS requested it “place all of its stock of midazolam — equivalent to around two year’s forecasted supply — into its wholesale partners”, even though the manufacturer “does not currently have any NHS contracts in England” to supply the drug.
“As a result of the NHS request [in March 2020], we are subsequently out of stock,” said Peter Kelly, managing director of Accord Healthcare.
However, he added that the Medicines and Healthcare products Regulatory Agency (MHRA) had given the manufacturer approval “for some French label stock — another 22,000 packs — to be sold into the NHS and [we] are currently waiting for the MHRA’s direction on where to place the stock”.
The manufacturer said the French stock only includes midazolam at the strength of 1mg/mL in 5mL, while the initial supply in March 2020 contained a variety of four different strengths.
A spokesperson for the DHSC said it was “working closely with industry, the NHS and others in the supply chain to help ensure patients can access the medicines they need and precautions are in place to reduce the likelihood of future shortages”.
The DHSC confirmed that its request for additional midazolam stock from Accord Healthcare was one of these precautions.
“As part of our national efforts to respond to the coronavirus outbreak, we are doing everything we can to ensure patients continue to access safe and effective medicines,” they added.