Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus
- PMID: 22536382
- PMCID: PMC3335060
- DOI: 10.1371/journal.pone.0035421
Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus
Erratum in
- PLoS One. 2012;7(8). doi:10.1371/annotation/2965cfae-b77d-4014-8b7b-236e01a35492
Abstract
Background: Severe acute respiratory syndrome (SARS) emerged in China in 2002 and spread to other countries before brought under control. Because of a concern for reemergence or a deliberate release of the SARS coronavirus, vaccine development was initiated. Evaluations of an inactivated whole virus vaccine in ferrets and nonhuman primates and a virus-like-particle vaccine in mice induced protection against infection but challenged animals exhibited an immunopathologic-type lung disease.
Design: Four candidate vaccines for humans with or without alum adjuvant were evaluated in a mouse model of SARS, a VLP vaccine, the vaccine given to ferrets and NHP, another whole virus vaccine and an rDNA-produced S protein. Balb/c or C57BL/6 mice were vaccinated i.m. on day 0 and 28 and sacrificed for serum antibody measurements or challenged with live virus on day 56. On day 58, challenged mice were sacrificed and lungs obtained for virus and histopathology.
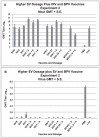
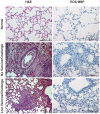
Results: All vaccines induced serum neutralizing antibody with increasing dosages and/or alum significantly increasing responses. Significant reductions of SARS-CoV two days after challenge was seen for all vaccines and prior live SARS-CoV. All mice exhibited histopathologic changes in lungs two days after challenge including all animals vaccinated (Balb/C and C57BL/6) or given live virus, influenza vaccine, or PBS suggesting infection occurred in all. Histopathology seen in animals given one of the SARS-CoV vaccines was uniformly a Th2-type immunopathology with prominent eosinophil infiltration, confirmed with special eosinophil stains. The pathologic changes seen in all control groups lacked the eosinophil prominence.
Conclusions: These SARS-CoV vaccines all induced antibody and protection against infection with SARS-CoV. However, challenge of mice given any of the vaccines led to occurrence of Th2-type immunopathology suggesting hypersensitivity to SARS-CoV components was induced. Caution in proceeding to application of a SARS-CoV vaccine in humans is indicated.
Conflict of interest statement
Figures







Similar articles
-
Effects of Toll-like receptor stimulation on eosinophilic infiltration in lungs of BALB/c mice immunized with UV-inactivated severe acute respiratory syndrome-related coronavirus vaccine.J Virol. 2014 Aug;88(15):8597-614. doi: 10.1128/JVI.00983-14. Epub 2014 May 21. J Virol. 2014. PMID: 24850731 Free PMC article.
-
Receptor-binding domain of SARS-CoV spike protein induces long-term protective immunity in an animal model.Vaccine. 2007 Apr 12;25(15):2832-8. doi: 10.1016/j.vaccine.2006.10.031. Epub 2006 Oct 30. Vaccine. 2007. PMID: 17092615 Free PMC article.
-
Severe acute respiratory syndrome-associated coronavirus vaccines formulated with delta inulin adjuvants provide enhanced protection while ameliorating lung eosinophilic immunopathology.J Virol. 2015 Mar;89(6):2995-3007. doi: 10.1128/JVI.02980-14. Epub 2014 Dec 17. J Virol. 2015. PMID: 25520500 Free PMC article.
-
Gold nanoparticle-adjuvanted S protein induces a strong antigen-specific IgG response against severe acute respiratory syndrome-related coronavirus infection, but fails to induce protective antibodies and limit eosinophilic infiltration in lungs.Microbiol Immunol. 2020 Jan;64(1):33-51. doi: 10.1111/1348-0421.12754. Epub 2019 Nov 18. Microbiol Immunol. 2020. PMID: 31692019 Free PMC article.
-
Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus.Avian Pathol. 2003 Dec;32(6):567-82. doi: 10.1080/03079450310001621198. Avian Pathol. 2003. PMID: 14676007 Free PMC article. Review.
Cited by 295 articles
-
COVID-19 Vaccination: The Mainspring of Challenges and the Seed of Remonstrance.Vaccines (Basel). 2021 Dec 13;9(12):1474. doi: 10.3390/vaccines9121474. Vaccines (Basel). 2021. PMID: 34960220 Free PMC article. Review.
-
Induction of Th1 and Th2 in the protection against SARS-CoV-2 through mucosal delivery of an adenovirus vaccine expressing an engineered spike protein.Vaccine. 2021 Dec 17:S0264-410X(21)01635-2. doi: 10.1016/j.vaccine.2021.12.024. Online ahead of print. Vaccine. 2021. PMID: 34952759 Free PMC article.
-
Nature of Acquired Immune Responses, Epitope Specificity and Resultant Protection from SARS-CoV-2.J Pers Med. 2021 Nov 25;11(12):1253. doi: 10.3390/jpm11121253. J Pers Med. 2021. PMID: 34945725 Free PMC article. Review.
-
Development of a SARS-CoV-2 Vaccine Candidate Using Plant-Based Manufacturing and a Tobacco Mosaic Virus-like Nano-Particle.Vaccines (Basel). 2021 Nov 17;9(11):1347. doi: 10.3390/vaccines9111347. Vaccines (Basel). 2021. PMID: 34835278 Free PMC article.
-
The Importance of RNA-Based Vaccines in the Fight against COVID-19: An Overview.Vaccines (Basel). 2021 Nov 17;9(11):1345. doi: 10.3390/vaccines9111345. Vaccines (Basel). 2021. PMID: 34835276 Free PMC article. Review.
References
-
- World Health Organization website. 2003. Available: http://www.who.int/csr/media/sars_wha.pdf. Accessed 2012 Apr 2. Severe acute respiratory syndrome (SARS): Status of the outbreak and lessons for the immediate future; unmasking a new disease. CSR/WHO, Geneva. 20 May 2003.
-
- Tsang KW, Ho PL, Ooi CG, Yee WK, Wang T, et al. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1953–66. - PubMed
-
- Poutanen SM, Low D, Henry B, Finkelstein S, Rose D, et al. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348:1953–66. - PubMed
-
- World Health Organization Website. Available: http://www.who.int/csr/sars/country/2003_04_04/en/index.html. Accessed 2004 April 21.
-
- Lee N, Hui D, Wu A, Chan P, Cameron P, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
